Benefits

Easy to use
Painless shallow nasal swab
Easy to follow instructions and video
Quick Results
Results in 15 mins

Accurate
Very high sensitivity
(clinical sensitivity of over 95% PPA)
Storage
15-month shelf life
Room temperature storage (2-30)

Internationally Accepted Test
Accepted and used widely around the world including USA, UK, and approved by the FDA and NHS
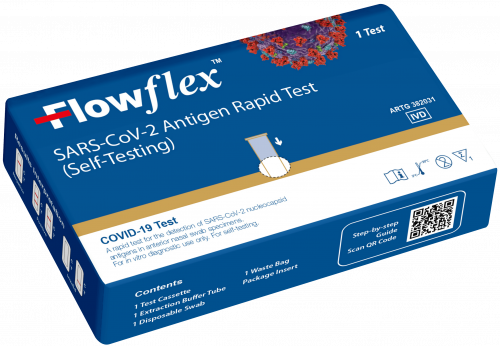
Easy and convenient self-test for SARS-CoV-2
Keep your family and business safe with the Flowflex™ SARS-CoV-2 Antigen Rapid Self-Test. Performed in just 15 minutes, simply swab, mix with reagents conveniently included in the kit, add the sample to the cassette, and wait 15 minutes to read the results.
Instructional Video
ALWAYS READ THE LABEL AND FOLLOW THE DIRECTIONS FOR USE.
Step-by-step Guide
ALWAYS READ THE LABEL AND FOLLOW THE DIRECTIONS FOR USE.
Failure to follow the instructions may result in inaccurate test
results. Read the Instructions for Use for full details.
01
Preparation
02
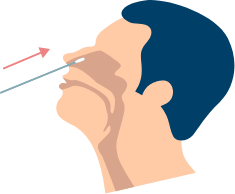
Test Procedure
03
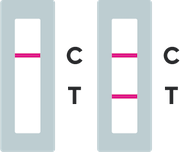
Results
04

Finish
Frequently Asked Questions
Corona viruses are a large family of viruses which may cause illness in animals or humans. In humans, several corona viruses are known to cause respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The most recently discovered coronavirus causes coronavirus disease COVID-19.
The most common symptoms of COVID-19 are fever, dry cough, and tiredness. Some patients may have aches and pains, nasal congestion, sore throat, or diarrhea. These symptoms are usually mild and begin gradually. Some people become infected but only have very mild symptoms. Most people (about 80%) recover from the disease without needing hospital treatment. Around 1 out of every 5 people who get COVID-19 becomes seriously ill and develops difficulty breathing. Older people, and those with underlying medical problems like high blood pressure, heart and lung problems, diabetes, or cancer, are at higher risk of developing serious illness. However, anyone can catch COVID-19 and become seriously ill. Even people with very mild symptoms of COVID-19 can transmit the virus. People of all ages who experience fever, cough and difficulty breathing should seek medical attention.
Potential risks include:
• Possible discomfort during sample collection.
• Possible incorrect test results (see Result Interpretation section).
Potential benefits include:
• The results, along with other information, can help you and your healthcare provider make informed decisions about your care.
• The results of this test may help limit the spread of COVID-19 to your family and others in your community.
The performance of Flowflex™ COVID-19 Antigen Rapid Test was established in an all-comers clinical study conducted between March 2021 and May 2021 with 172 nasal swabs self-collected or pair-collected by another study participant from 108 individual symptomatic patients (within 7 days of onset) suspected of COVID-19 and 64 asymptomatic patients. All subjects were screened for the presence or absence of COVID-19 symptoms within two weeks of study enrolment. The Flowflex™ COVID-19 Antigen Rapid Test was compared to an FDA authorised molecular SARS-CoV-2 test. The Flowflex™ COVID-19 Antigen Rapid Test correctly identified 93% of positive specimens and 100% of negative specimens.
A negative test result indicates no antigens for COVID-19 were detected. It is possible for this test to give a negative result that is incorrect (false negative) in some people with COVID-19 and negative results are presumptive and may need to be confirmed with a molecular test. This means that you could possibly still have COVID-19 even though the test is negative. If you test negative and continue to experience symptoms of fever, cough and/or shortness of breath you should seek follow up care with your healthcare provider immediately. Your healthcare provider may suggest you need another test to determine if you have contracted the virus causing COVID-19. If you are concerned about your COVID-19 infection status after testing or think you may need follow up testing, please contact your healthcare provider.
The known limitations of the SARS-CoV-2 Antigen Rapid Test are:
• The SARS-CoV-2 Antigen Rapid Test is for in vitro diagnostic use only. The test should be used for the detection of SARS-CoV-2 antigens in nasal swab specimens only. The intensity of the test line does not necessarily correlate to SARS-CoV-2 viral titer in the specimen.
• Specimens should be tested as quickly as possible after specimen collection and at most within the hour following collection.
• Use of viral transport media may result in decreased test sensitivity.
• A false-negative test may result if the level of antigen in a sample is below the detection limit of the test or if the sample was collected incorrectly.
• Test results should be correlated with other clinical data available to the physician.
• A positive test result does not rule out co-infections with other pathogens.
• A positive test result does not differentiate between SARS-CoV and SARS-CoV-2.
• A negative test result is not intended to rule out other viral or bacterial infections.
• A negative result, from a patient with symptom onset beyond seven days, should be treated as presumptive and confirmed with a molecular assay, if necessary, for clinical management.
• If the differentiation of specific SARS viruses and strains is needed, additional testing is required.
COVID-19 is the infectious disease caused by the most recently discovered coronavirus. This new virus and disease were unknown before. COVID-19 is now a pandemic affecting many countries globally.
No, this nasal swab does not hurt because it is not required to be inserted deep in the nasal cavity as in the case of a nasopharyngeal swab usually taken in the case of PCR testing. In addition, the nasal swab is not sharp, and it should not hurt. Sometimes the swab can feel slightly uncomfortable. If you feel pain, please stop the test and seek advice from your healthcare provider.
There are different kinds of tests for COVID-19. Molecular tests (also known as PCR tests) detect genetic material from the virus. Antigen tests, such as the Flowflex™ COVID-19 Antigen Rapid Test detect proteins from the virus. Antigen tests are very specific for the COVID-19 virus but are not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection. If your test result is negative, you should discuss with your healthcare provider whether an additional molecular test is necessary and if you should continue isolating at home.
Results are for the identification of SARS-CoV-2 nucleocapsid antigen. This antigen is generally detectable in upper respiratory samples during the acute phase of infection. Positive results
indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results from patients more than seven days post symptom onset should be treated as presumptive and confirmed with a molecular assay, if necessary, for patient management. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
A positive test result means that antigens from COVID-19 were detected and it is very likely you currently have COVID-19 disease. Self-isolate at home as per your local state regulations to avoid spreading the virus to other people and consult your healthcare provider as soon as possible. Consult your GP for confirmatory testing and follow-up clinical care.
How It Works
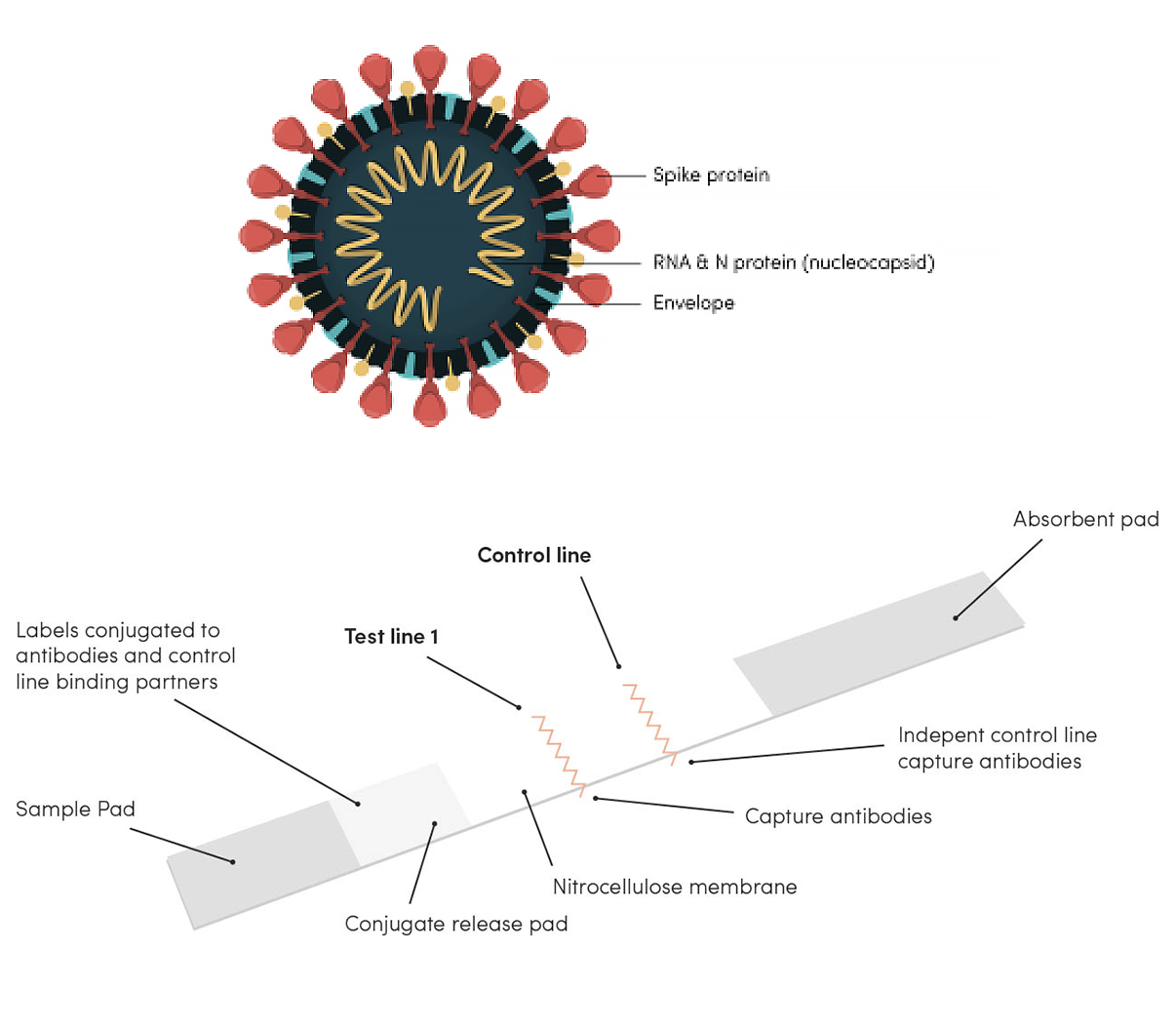
Flowflex™ is a lateral flow assay that detects the presence or absence of the SARS-CoV-2 nucleocapsid antigen, the protein that encapsulates SARS-CoV-2 RNA (genetic material) within the virus.
The lateral flow assay (LFA), also known as lateral flow immunoassay, uses antibodies labelled with an enzyme that emits colour to detect a specific target.
- The sample is loaded onto the sample pad which controls the flow of the sample through the rest of the cassette.
- The sample will flow through the conjugate pad which contains the labelled antibodies. If SARS-CoV-2 is present, the labelled antibodies will bind to SARS-CoV-2 in the sample and continue to migrate along the test.
- The sample will continue to move along the nitrocellulose membrane where binding reagents will bind to the target at the test line. If SARS-CoV-2 is present, a coloured line will form. How clearly the line shows up depends on the amount of SARS-CoV-2 present. The control line should also form if the assay was correctly performed. If there is no formation of the control line, the test is not valid.
- The absorbent pad at the end absorbs any excess sample.
Corona viruses are a large family of viruses which may cause illness in animals or humans. In humans, several corona viruses are known to cause respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The most recently discovered coronavirus causes coronavirus disease COVID-19.
COVID-19 is the infectious disease caused by the most recently discovered coronavirus. This new virus and disease were unknown before. COVID-19 is now a pandemic affecting many countries globally.
The most common symptoms of COVID-19 are fever, dry cough, and tiredness. Some patients may have aches and pains, nasal congestion, sore throat, or diarrhea. These symptoms are usually mild and begin gradually. Some people become infected but only have very mild symptoms. Most people (about 80%) recover from the disease without needing hospital treatment. Around 1 out of every 5 people who get COVID-19 becomes seriously ill and develops difficulty breathing. Older people, and those with underlying medical problems like high blood pressure, heart and lung problems, diabetes, or cancer, are at higher risk of developing serious illness. However, anyone can catch COVID-19 and become seriously ill. Even people with very mild symptoms of COVID-19 can transmit the virus. People of all ages who experience fever, cough and difficulty breathing should seek medical attention.
No, this nasal swab does not hurt because it is not required to be inserted deep in the nasal cavity as in the case of a nasopharyngeal swab usually taken in the case of PCR testing. In addition, the nasal swab is not sharp, and it should not hurt. Sometimes the swab can feel slightly uncomfortable. If you feel pain, please stop the test and seek advice from your healthcare provider.
Potential risks include:
• Possible discomfort during sample collection.
• Possible incorrect test results (see Result Interpretation section).
Potential benefits include:
• The results, along with other information, can help you and your healthcare provider make informed decisions about your care.
• The results of this test may help limit the spread of COVID-19 to your family and others in your community.
There are different kinds of tests for COVID-19. Molecular tests (also known as PCR tests) detect genetic material from the virus. Antigen tests, such as the Flowflex™ COVID-19 Antigen Rapid Test detect proteins from the virus. Antigen tests are very specific for the COVID-19 virus but are not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection. If your test result is negative, you should discuss with your healthcare provider whether an additional molecular test is necessary and if you should continue isolating at home.
The performance of Flowflex™ COVID-19 Antigen Rapid Test was established in an all-comers clinical study conducted between March 2021 and May 2021 with 172 nasal swabs self-collected or pair-collected by another study participant from 108 individual symptomatic patients (within 7 days of onset) suspected of COVID-19 and 64 asymptomatic patients. All subjects were screened for the presence or absence of COVID-19 symptoms within two weeks of study enrolment. The Flowflex™ COVID-19 Antigen Rapid Test was compared to an FDA authorised molecular SARS-CoV-2 test. The Flowflex™ COVID-19 Antigen Rapid Test correctly identified 93% of positive specimens and 100% of negative specimens.
Results are for the identification of SARS-CoV-2 nucleocapsid antigen. This antigen is generally detectable in upper respiratory samples during the acute phase of infection. Positive results
indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results from patients more than seven days post symptom onset should be treated as presumptive and confirmed with a molecular assay, if necessary, for patient management. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
A negative test result indicates no antigens for COVID-19 were detected. It is possible for this test to give a negative result that is incorrect (false negative) in some people with COVID-19 and negative results are presumptive and may need to be confirmed with a molecular test. This means that you could possibly still have COVID-19 even though the test is negative. If you test negative and continue to experience symptoms of fever, cough and/or shortness of breath you should seek follow up care with your healthcare provider immediately. Your healthcare provider may suggest you need another test to determine if you have contracted the virus causing COVID-19. If you are concerned about your COVID-19 infection status after testing or think you may need follow up testing, please contact your healthcare provider.
A positive test result means that antigens from COVID-19 were detected and it is very likely you currently have COVID-19 disease. Self-isolate at home as per your local state regulations to avoid spreading the virus to other people and consult your healthcare provider as soon as possible. Consult your GP for confirmatory testing and follow-up clinical care.
The known limitations of the SARS-CoV-2 Antigen Rapid Test are:
• The SARS-CoV-2 Antigen Rapid Test is for in vitro diagnostic use only. The test should be used for the detection of SARS-CoV-2 antigens in nasal swab specimens only. The intensity of the test line does not necessarily correlate to SARS-CoV-2 viral titer in the specimen.
• Specimens should be tested as quickly as possible after specimen collection and at most within the hour following collection.
• Use of viral transport media may result in decreased test sensitivity.
• A false-negative test may result if the level of antigen in a sample is below the detection limit of the test or if the sample was collected incorrectly.
• Test results should be correlated with other clinical data available to the physician.
• A positive test result does not rule out co-infections with other pathogens.
• A positive test result does not differentiate between SARS-CoV and SARS-CoV-2.
• A negative test result is not intended to rule out other viral or bacterial infections.
• A negative result, from a patient with symptom onset beyond seven days, should be treated as presumptive and confirmed with a molecular assay, if necessary, for clinical management.
• If the differentiation of specific SARS viruses and strains is needed, additional testing is required.
How It Works
Flowflex™ is a lateral flow assay that detects the presence or absence of the SARS-CoV-2 nucleocapsid antigen, the protein that encapsulates SARS-CoV-2 RNA (genetic material) within the virus.
The lateral flow assay (LFA), also known as lateral flow immunoassay, uses antibodies labelled with an enzyme that emits colour to detect a specific target.
- The sample is loaded onto the sample pad which controls the flow of the sample through the rest of the cassette.
- The sample will flow through the conjugate pad which contains the labelled antibodies. If SARS-CoV-2 is present, the labelled antibodies will bind to SARS-CoV-2 in the sample and continue to migrate along the test.
- The sample will continue to move along the nitrocellulose membrane where binding reagents will bind to the target at the test line. If SARS-CoV-2 is present, a coloured line will form. How clearly the line shows up depends on the amount of SARS-CoV-2 present. The control line should also form if the assay was correctly performed. If there is no formation of the control line, the test is not valid.
- The absorbent pad at the end absorbs any excess sample.

| Test Format | Cassette |
| Sample Format | Anterior nasal swab samples |
| Detects | Nucleocapsid protein antigen from SARS-CoV-2 |
| Sample Volume | 4 drops of the prepared specimen |
| Relative Sensitivity | 97.1% (95% CI: 93.1%-98.9%) 95% Confidence Intervals |
| Relative Specificity | 99.5% (95% CI: 98.2%-99.9%) 95% Confidence Intervals |
| Test Time | 15 minutes |
| Shelf Life | 15 Months |
| Storage Temperature | 2 – 30°C |



